VitaPCR™ AT A GLANCE
VitaPCR™ is a molecular point-of-care platform based on fast Real Time-PCR for rapid molecular detection, directly from oro and nasopharingeal swabs, of SARSCoV-2 and other respiratory viruses. The system combines high sensitivity with ease of use, delivering accurate and actionable results in just 20 minutes, exactly where they are needed.
PRINCIPLE OF THE TESTS
All assays running on the VitaPCR system are based on fast Real Time PCR with multicolor fluorescence detection in up to four different channels. Sample preparation includes single step lysis and extraction solutions to allow an efficient and rapid release of nucleic acids. This combination ensures sensitivity levels that closely compare to laboratory PCR tests and time to results that closely compare to rapid antigen tests.
CONVENIENT AND EFFICIENT SOLUTION
VitaPCR can be installed in minutes, doesn’t require additional laboratory equipment and thanks to the rapid turnaround time can process up to 3 samples per hour with the possibility to add more instruments working in parallel when throughput need exceeds the capacity of a single unit. Using the VitaDataLink software it is possible to connect up to 8 units to a single PC to simplify data management and connect the hub to a central LIS system.
VITAPCR KEY BENEFITS
EASY
Sample preparation is completed in 1 minute just introducing the swab into the collection buffer. In a few seconds the sample is ready to be transferred to the PCR tube.
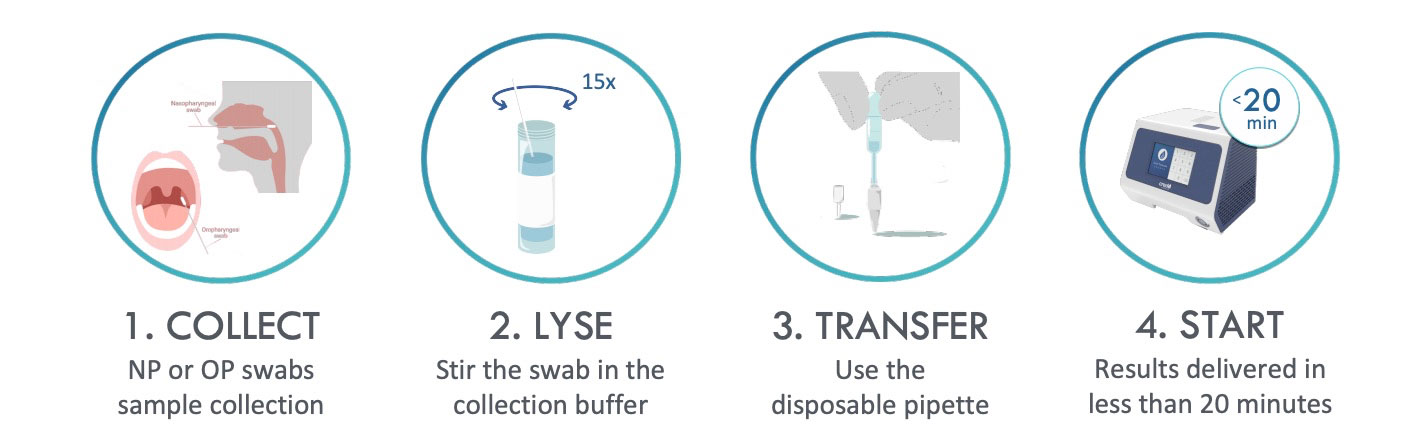
RAPID
Results are ready in 20 minutes, for both positive and negative samples.
SENSITIVE
Based on Real Time PCR, the assay provides very high sensitvity, allowing to detect patients with moderate to low viral load.
INTUITIVE
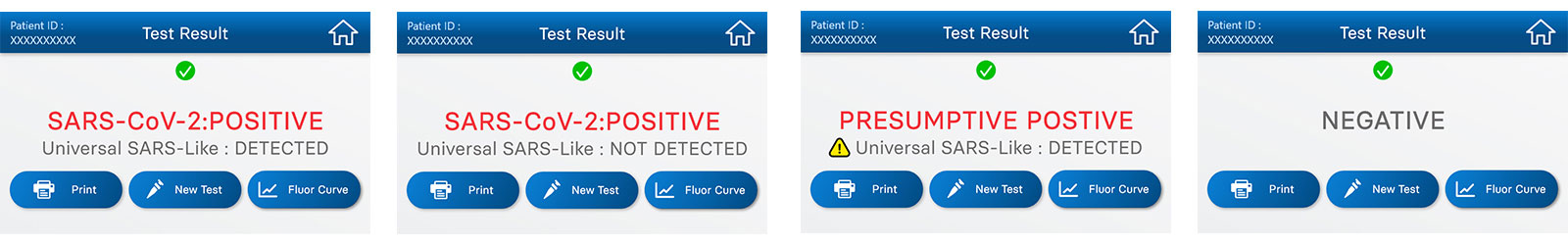
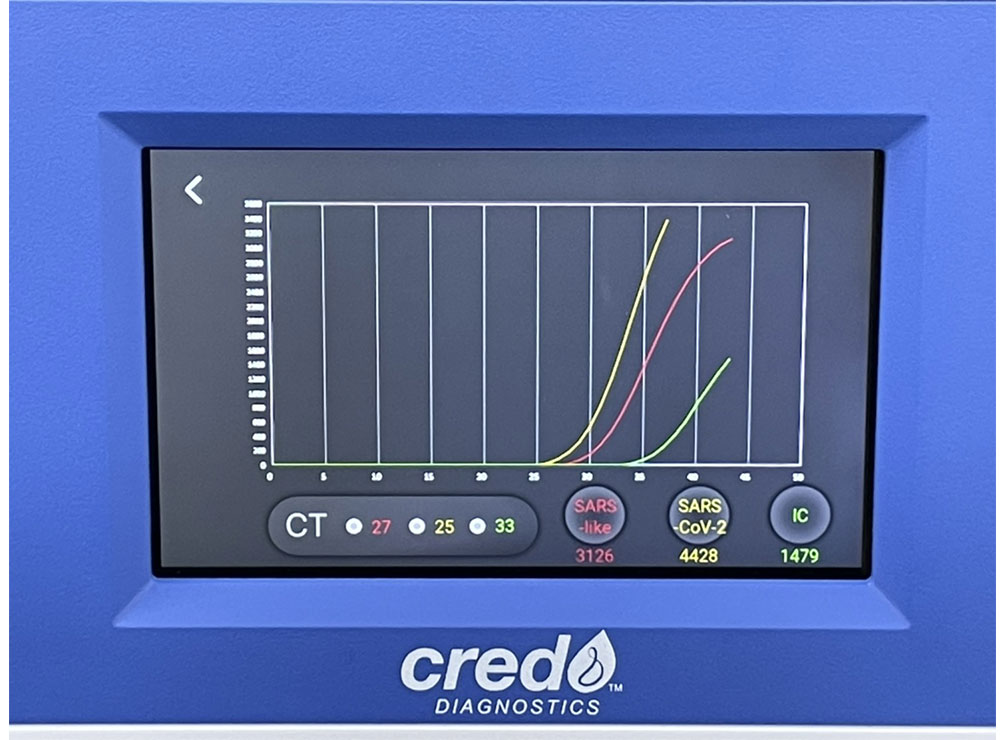
The instrument software automatically interprets results and shows all details of the run, including curve graphics and Ct values, by means of its intuitive, user interface.
VERSATILE
The system wheighs only 1.2 Kg with very small footprint. It can be deployed anywhere and does not require additional equipment to be used. Assay reagents are for room temperature storage.
CONNECTED
VitaDataLink connection pack supports the HL7 messaging standard, allowing to interface VitaPCR™ to LIS, middleware, EMR and point of care data manager.
VitaPCR™ Product List for Orders
CODE DESCRIPTION
- 51572 VitaPCR™ Instrument
- 52957 SARS-CoV-2 Gen2 Assay
- 51574 SARS-CoV-2 (Gen1) Assay
- 51573 Flu A+B Assay
- 52182 Influenza/SARS-CoV-2 Assay
- 52253 Strep A Assay
- 52254 Flu/RSV Assay
- 52289 SARS-CoV-2 Gen2 External Control Set
- 51699 SARS-CoV-2 (Gen1) External Control Set
- 54373 Thermal Printer
- 52207 Rack (tube holder)
BROCHURE AVAILABLE HERE:
